FOB Price:Get Latest Price
Min.Order Quantity:10 Piece/Pieces
Supply Ability:4000 Piece/Pieces per Month
Port:DALIAN
Payment Terms:L/C,T/T
Overview
YINYI® Polymer-free Drug-coated (Paclitaxel) Coronary Stent System is a new generation DES self-developed by Liaoning Biomedical Materials R&D Center Co., Ltd. It applies an innovative method for drug loading, which provides a safe, effective and economic therapy for the patients with coronary artery stenosis.
Feature
The traditional DES applies a polymer as a carrier for drug, but with the widely used in clinic, there are much problems occurred:
Late stent thrombosis due to the delayed healing
Late catch-up phenomenon of restenosis due to the chronic stimulation and inflammatory response caused by polymer
Late acquired stent malapposition, LASM
More risk of bleeding due to the long-term antiplatelet drug taken
The ideal way to solve these problems is to develop a new generation stent, a polymer-free DES.
YINYI applies an innovative concept for drug loading. Many micropores on the stent surface can load paclitaxel (a lipophilic drug), and make the best combination between drug release and inhibition of smooth muscle cells (SMC). Without any polymer or its stimulation, endothelial cells are easier to cover the stent, so a healthy endothelialization can be completed quickly, and shorter the antiplatelet therapy.
Innovative method for drug loading with micropores
Realized by the patent technology
Depth of pore: < 500nm, only 1/200 of the stent thickness
Diameter of pore: 1~2μm, thousand times larger than paclitaxel molecule (<1nm)

Effective drug release
The micropores and lipophilic paclitaxel make the best combination between drug release and inhibition of smooth muscle cells.
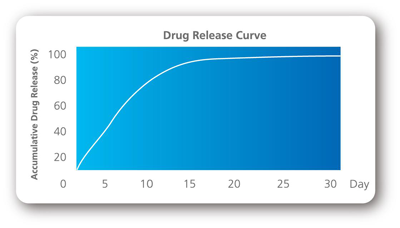
- 10days 72%
- 30days 95%
- 60days 100%
- After 60 days = 100% BMS
Natural drug
As a natural extract from Taxus, paclitaxel is a first line antitumor drug. Many clinical data have proved that paclitaxel-eluting stent has more efficacy than sirolimus-eluting stent in the following therapy:
Coronary artery disease with diabetes mellitus
In-stent restenosis (ISR) after sirolimus-eluting stent is implanted
Patient allergic to sirolimus
Higher cost performance
YINYI provides a higher cost performance for patients:
An upgrade and substitution product of polymer-based DES
Better effect in the therapy of coronary artery disease with diabetes mellitus
Save the expenses for patients as a shorter DAPT is available
Clinical Use
YINYI has proved its safety and efficacy through several large-scale clinical trials and real-world widely use:
1 pre-clinical trial (about 170 patients)
2 large-scale clinical trials (1045/1626 patients)
6 years implanted in 150,000 patients
400 hospitals use YINYI
The top one polymer-free DES used in the world